Ethanol is most commonly consumed as a popular recreational drug it is a psychoactive substance and is the principal type of alcohol found in alcoholic drinks.
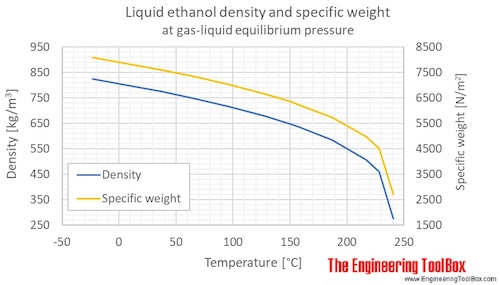
Density of ethanol at room temperature.
Methyl alcohol ethyl alcohol and isopropyl alcohol are free flowing liquids with fruity odours.
If the object floats on water it means the density of the object is less than the density of water and if the object sinks it means that its density is more than.
The calculator below can be used to estimate the density and specific weight of liquid ethanol at given temperature.
V is the volume of the substance.
Density of ethanol at room temperature.
Specific weight is given as n m 3 and lb f ft 3.
The density of ethanol at room temperature 0 789 g ml please show work.
Overall the keto enol tautomerization occurs slowly but is catalyzed by acids.
We have the density and the volume so we can find the mass.
Phase behavior triple point.
The equilibrium constant is 6 10 7 at room temperature thus that the relative amount of the enol form in a sample of acetaldehyde is very small.
1 answer nam d.
Remember that density is given by.
Online ethanol density calculator.
The logic is to divide the value of kg m 3 by 1000 to get pure water density in g ml.
Density is defined as the mass per unit of volume.
It basically depends on how closely the atoms are spaced in a particular object or liquid.
Most of the common alcohols are colourless liquids at room temperature.
The higher alcohols those containing 4 to 10 carbon atoms are somewhat viscous or oily and they have heavier fruity odours.
The output density is given as kg m 3 lb ft 3 lb gal us liq and sl ft 3.
The table was taken from perry s chemical engineers handbook by robert h.
Jan 15 2018 48 9 grams of ethanol.
The density of ethanol at room temperature 0 789 g ml.
At room temperature i e 22 c the density of water in kg m 3 is 997 77.
514 k 241 c 63 bar std enthalpy change of fusion δ fus h o 4 9 kj mol.
150 k 123 c 0 00043 pa critical point.
Help me please please please please please this is chem hw i dont know how to do i think its m d v but am not sure the pronlem once again is what is the mass of 63 5 ml of ethonal.
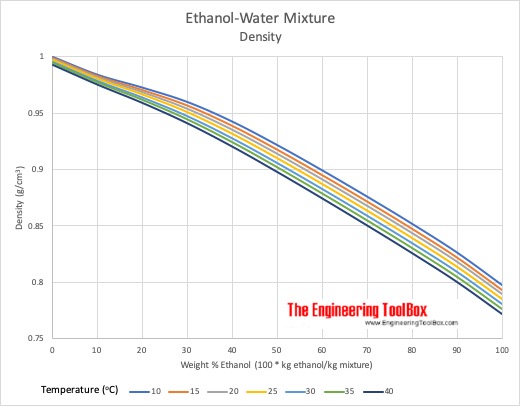
This is a table of density kg l and the corresponding concentration weight or volume of ethanol c 2 h 5 oh in water at a temperature of 20 c.
Ethanol ethyl alcohol c 2 h 5 oh is a volatile flammable colorless liquid with a slight characteristic odor it is produced via petrochemical processes or naturally by the fermentation of sugars by yeasts.
Alcohol alcohol physical properties of alcohols.
In other words at the same temperature the density of water in g ml or g cm 3 is 0 99777.